
There is a great range in the explosivity of volcanic eruptions. Many eruptions are relatively quiescent and are characterized by the calm, nonviolent extrusion of lava flows on the earth's surface. Other eruptions, however, are highly explosive and are characterized by the violent ejection of fragmented volcanic debris, called tephra, which can extend tens of kilometers into the atmosphere above the volcano.

|

|
|
with effusive lava flows |
voluminous plume of tephra |
Whether or not an eruption falls into one of these end-member types depends on a variety of factors, which are ultimately linked to the composition of the magma (molten rock) underlying the volcano. Magma composition is discussed below, followed by a description of the controlling factors on explosivity -- viscosity, temperature, and the amount of dissolved gases in the magma.
Only ten elements make up the bulk of most magmas: oxygen (O), silicon (Si), aluminum (Al), iron (Fe), magnesium (Mg), titanium (Ti) calcium (Ca), sodium (Na), potassium (K), and phosphorous (P). Because oxygen and silicon are by far the two most abundant elements in magma, it is convenient to describe the different magma types in terms of their silica content (SiO2). The magma types vary from mafic magmas, which have relatively low silica and high Fe and Mg contents, to felsic magmas, which have relatively high silica and low Fe and Mg contents. Mafic magma will cool and crystallize to produce the volcanic rock basalt, whereas felsic magma will crystallize to produce dacite and rhyolite. Intermediate-composition magmas will crystallize to produce the rock andesite. Because the mafic rocks are enriched in Fe and Mg, they tend to be darker colored than the felsic rock types.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
There also exists more unusual magmas that erupt less commonly on the Earth's surface as ultramafic, carbonatite, and strongly alkaline lavas.
For an historical note on rock terminology see: Basic/Acidic vs. Mafic/Felsic
The viscosity of a substance is a measure of its consistency. Viscosity is defined as the ability of a substance to resist flow. In a sense, viscosity is the inverse of fluidity. Cold molasses, for example, has a higher viscosity than water because it is less fluid. A magma's viscosity is largely controlled by its temperature, composition, and gas content (see downloadable programs at the bottom of this page). The effect of temperature on viscosity is intuitive. Like most liquids, the higher the temperature, the more fluid a substance becomes, thus lowering its viscosity.
Composition plays an even greater role in determining a magma's viscosity. A magma's resistance to flow is a function of its "internal friction" derived from the generation of chemical bonds within the liquid. Chemical bonds are created between negatively charged and positively charged ions (anions and cations, respectively). Of the ten most abundant elements found in magmas (see above), oxygen is the only anion. Silicon, on the other hand, is the most abundant cation. Thus, the Si-O bond is the single most important factor in determining the degree of a magma's viscosity. These two elements bond together to form "floating radicals" in the magma, while it is still in its liquid state (i.e., Si-O bonds begin to form well above the crystallization temperature of magma). These floating radicals contain a small silicon atom surrounded by four larger oxygen atoms (SiO4). This atomic configuration is in the shape of a tetrahedron. The radicals are therefore called silicon-oxygen tetrahedra, as shown here.
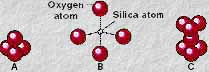
|
These floating tetrahedra are electrically charged compounds. As such, they they are electrically attracted to other Si-O tetrahedra. The outer oxygen atoms in each tetrahedron can share electrons with the outer oxygen atoms of other tetrahedra. The sharing of electrons in this manner results in the development of covalent bonds between tetrahedra. In this way Si-O tetrahedra can link together to form a variety shapes: double tetrahedra (shown here, C), chains of tetrahedra, double chains of tetrahedra, and complicated networks of tetrahedra. As the magma cools, more and more bonds are created, which eventually leads to the development of crystals within the liquid medium. Thus, the Si-O tetrahedra form the building blocks to the common silicate minerals found in all igneous rocks. However, while still in the liquid state, the bonding of tetrahedra results in the polymerization of the liquid, which increases the "internal friction" of the magma, so that it more readily resists flow. Magmas that have a high silica content will therefore exhibit greater degrees of polymerization, and have higher viscosities, than those with low-silica contents.
The amount of dissolved gases in the magma can also affect it's viscosity, but in a more ambiguous way than temperature and silica content. When gases begin to escape (exsolve) from the magma, the effect of gas bubbles on the bulk viscosity is variable. Although the growing gas bubbles will exhibit low viscosity, the viscosity of the residual liquid will increase as gas escapes. The overall bulk viscosity of the bubble-liquid mixture depends on both the size and distribution of the bubbles. Although gas bubbles do have an effect on the viscosity, the more important role of these exsolving volatiles is that they provide the driving force for the eruption. This is discussed in more detail below.
As dissolved gases are released from the magma, bubbles will begin to form. Bubbles frozen in a porous or frothy volcanic rock are called vesicles, and the process of bubble formation is called vesiculation or gas exsolution. The dissolved gases can escape only when the vapor pressure of the magma is greater than the confining pressure of the surrounding rocks. The vapor pressure is largely dependent on the amount of dissolved gases and the temperature of the magma.

|

|
|
vertical vesicle cylinders |
|
Explosive eruptions are initiated by vesiculation, which in turn, can be promoted in two ways: (1) by decompression, which lowers the confining pressure, and (2) by crystallization, which increases the vapor pressure. In the first case, magma rise can lead to decompression and the formation of bubbles, much like the decompression of soda and the formation of CO2 bubbles when the cap is removed. This is sometimes referred to as the first boiling. Alternatively, as magma cools and anhydrous minerals begin to crystallize out of the magma, the residual liquid will become increasingly enriched in gas. In this case, the increased vapor pressure in the residual liquid can also lead to gas exsolution. This is sometimes referred to as second (or retrograde) boiling. Both mechanisms can trigger an explosive volcanic eruption.
The amount of dissolved gas in the magma provides the driving force for explosive eruptions. The viscosity of the magma, however, is also an important factor in determining whether an eruption will be explosive or nonexplosive. A low-viscosity magma, like basalt, will allow the escaping gases to migrate rapidly through the magma and escape to the surface. However, if the magma is viscous, like rhyolite, its high polymerization will impede the upward mobility of the gas bubbles. As gas continues to exsolve from the viscous melt, the bubbles will be prevented from rapid escape, thus increasing the overall pressure on the magma column until the gas ejects explosively from the volcano. As a general rule, therefore, nonexplosive eruptions are typical of basaltic-to-andesitic magmas which have low viscosities and low gas contents, whereas explosive eruptions are typical of andesitic-to-rhyolitic magmas which have high viscosities and high gas contents.
|
|
TYPE |
(centigrade) |
|
CONTENT |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
There are, however, two exceptions to this general rule. Andesitic-to-rhyolitic lavas that have been degassed often erupt at the surface nonexplosively as viscous lava domes or obsidian flows. Similarly, many of the so-called hydrovolcanic eruptions involve basaltic-to-andesitic magmas that erupt explosively in the presence of groundwater or surface water. For more information on the variability of explosivity, see the Volcano Explosivity Index.
Intermediate to advanced users may be interested in the following programs for calculating viscosity. Click image to download.
![]() Viscosity for Windows. Developed
by Dr. Jon Dehn, this program calculates the viscosity of silicate
magmas from the magma composition and temperature.
Viscosity for Windows. Developed
by Dr. Jon Dehn, this program calculates the viscosity of silicate
magmas from the magma composition and temperature.
![]() Magma for Windows. Developed by Dr. Ken Wohletz, this program
calculates the density and viscosity of silicate magmas from the
magma composition and temperature.
Magma for Windows. Developed by Dr. Ken Wohletz, this program
calculates the density and viscosity of silicate magmas from the
magma composition and temperature.