
 |
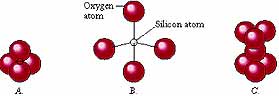
| Silicon-oxygen tetrahedron - These floating radicals are the building blocks for all silicate minerals. They begin to form well above the crystallization temperature of the magma. A small silicon atom bonds with four larger oxygen atoms to create a single tetrahedron (A and B). These chemical compounds (radicals) are electrically charged and can bond (covalently) with other tetrahedra (C). The linked tetrahedra can form a variety of different shapes, which include single chains of tetrahedra, double chains, and complex networks of interlinked tetrahedra. The bonding will increase polymerization as temperature falls and crystallization proceeds. The viscosity of the magma will increase with increasing silica content and increasing polymerization. Courtesy of JAK graphics and J. Wiley & Sons. |